Table of Contents
In participant-centric studies, participant data like name, age, sex, etc., is collected along with the specimens. The study can be -
...
| Expand | ||
|---|---|---|
| ||
|
Details for all the fields on the CP overview page,
...
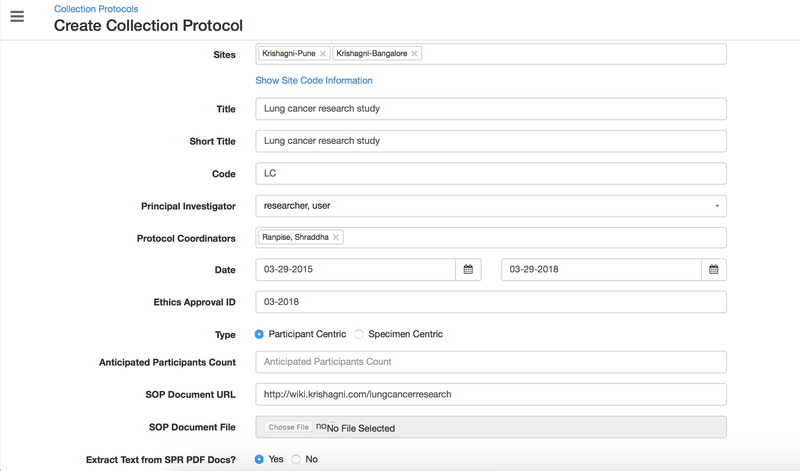
Sites
...
Cardiovascular diseases and risk factors knowledge and awareness in people with type 2 diabetes mellitus: a global evaluation
...
Short Title
...
Code
...
Principal Investigator
...
A specialized a researcher who supports the management and coordination of clinical research studies. There can be multiple coordinators selected.
...
Link to the SOP document uploaded on the university web portal
...
| Note | ||
|---|---|---|
| ||
|
...