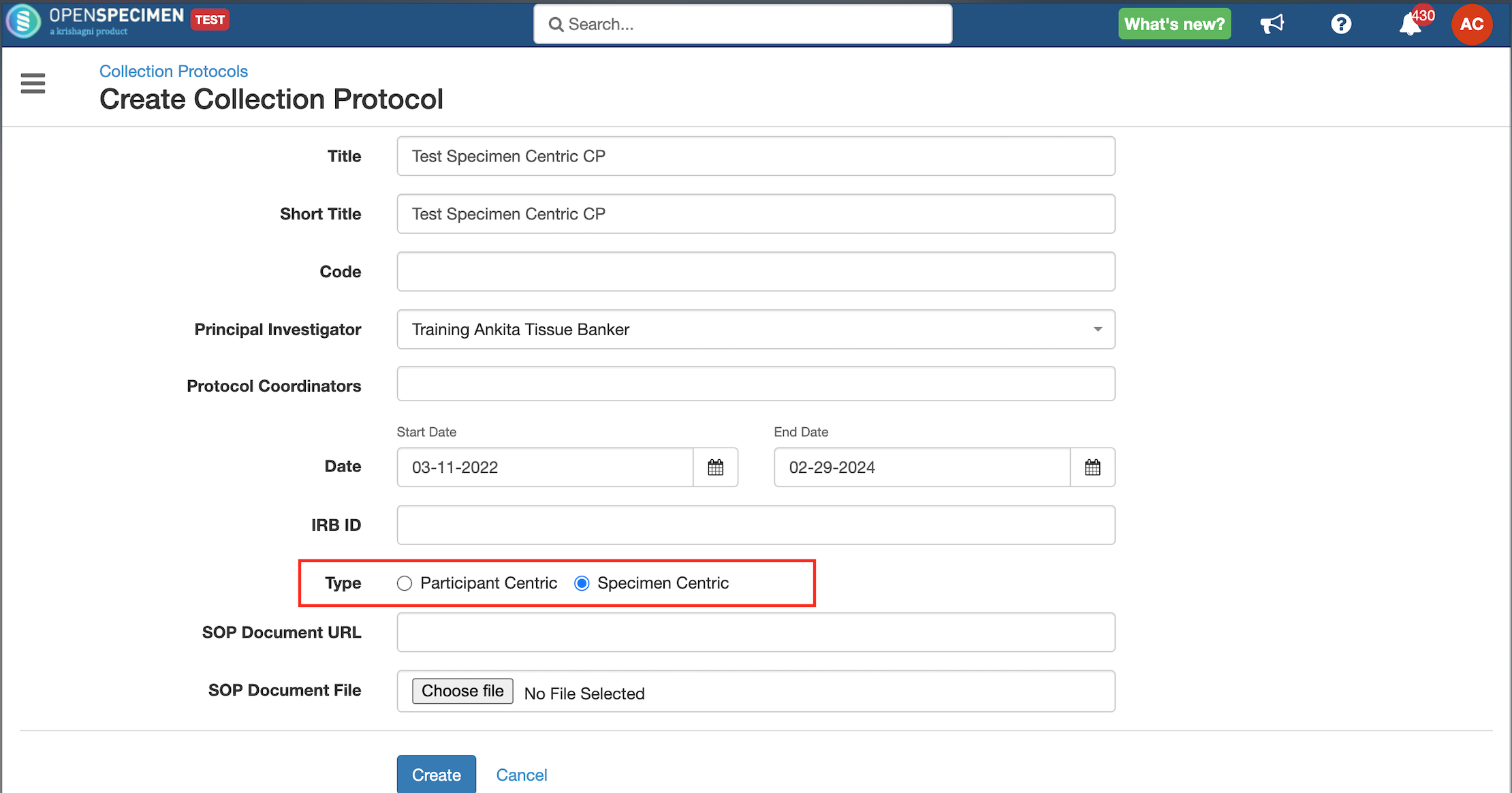
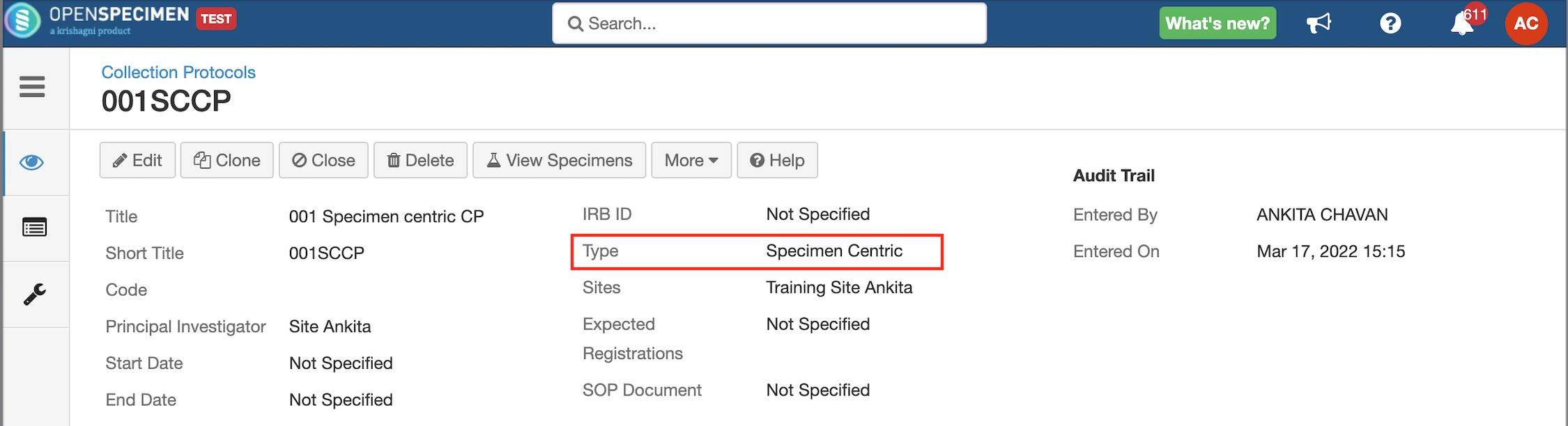
Some studies might be collecting only specimen information with no linkage to the participants. OpenSpecimen supports specimen-centric studies. During protocol set-up, administrators can choose if a study is a participant or a specimen-centric study.
Once a protocol is defined as 'Specimen Centric,' the 'Events' tab is hidden in the protocol set-up as there is no need to capture participants and visits.
When the user accesses such protocol for data collection, the specimens list page is directly displayed.
Option to create specimen directly is available without linking to participant or visit. You can add multiple specimens of the same or different types by clicking the 'Add Specimen' button.
Setting up Specimen Requirements (SR) (v9.0)
Specimen Requirements can be set up for specimen-centric CP.
Even though there is no planned study calendar for a specimen-centric CP, biobanks might be collecting specific types of specimens like Whole Blood, Urine, and Tissue. Once that is collected, the processing steps would remain the same based on the parent type of specimens. For example, 10 ml EDTA Whole Blood tubes are always processed into five aliquots of plasma and ten aliquots of buffy coat.
To support this use case within specimen-centric CPs, it is now possible to pre-define specimen requirements (SRs) similar to longitudinal CPs.
For more information about the SR please refer to the wiki page.