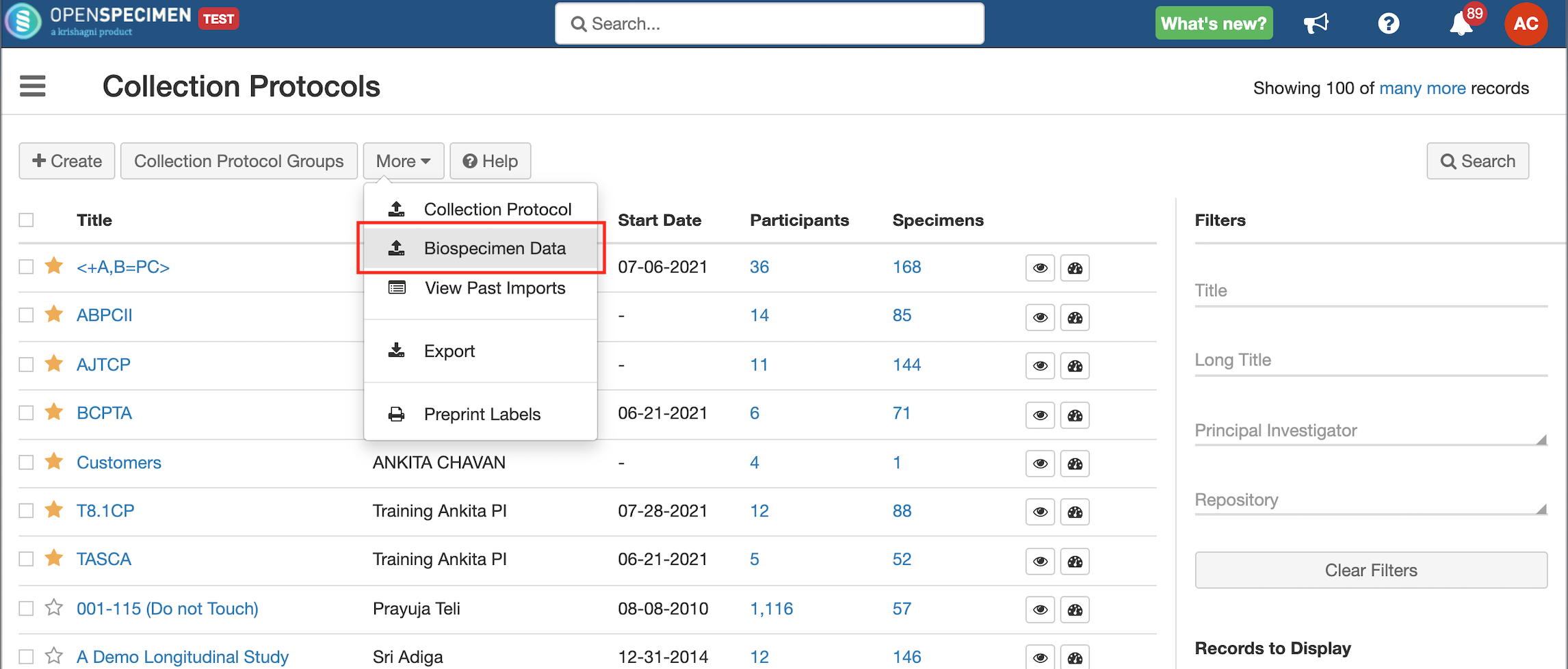
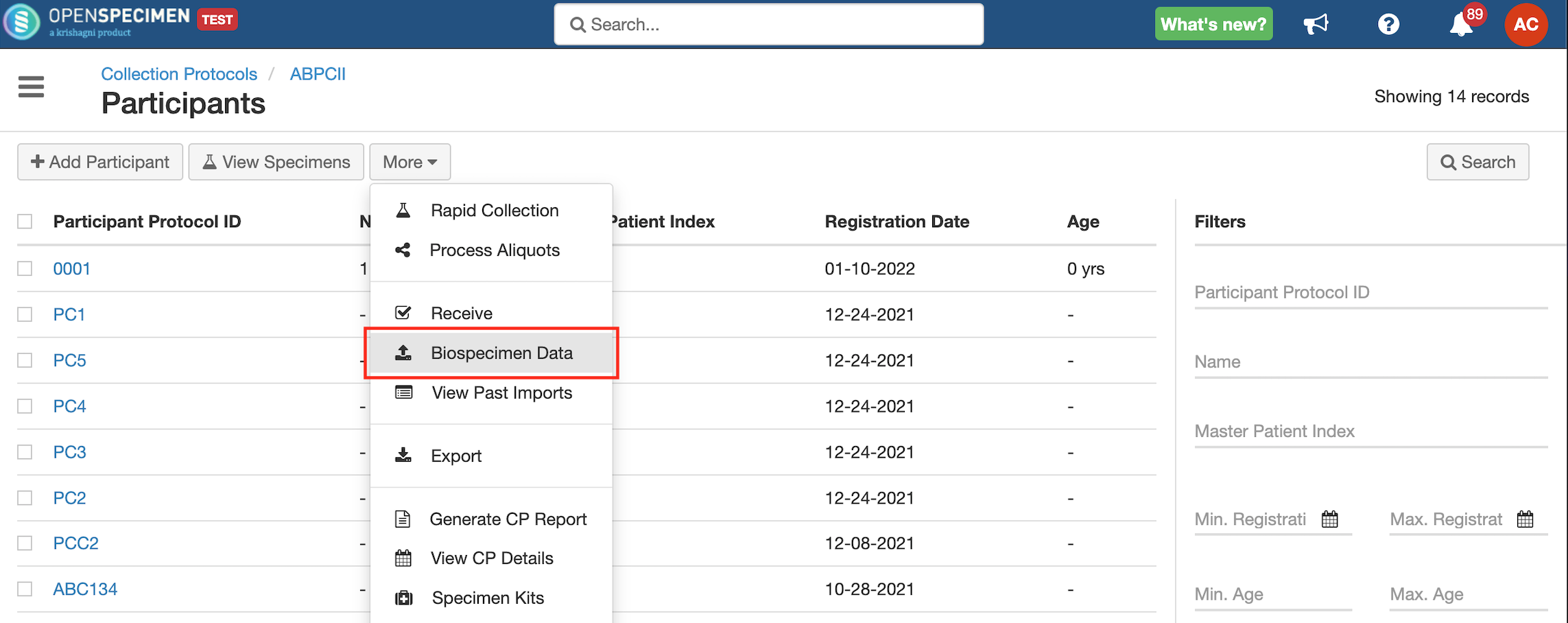
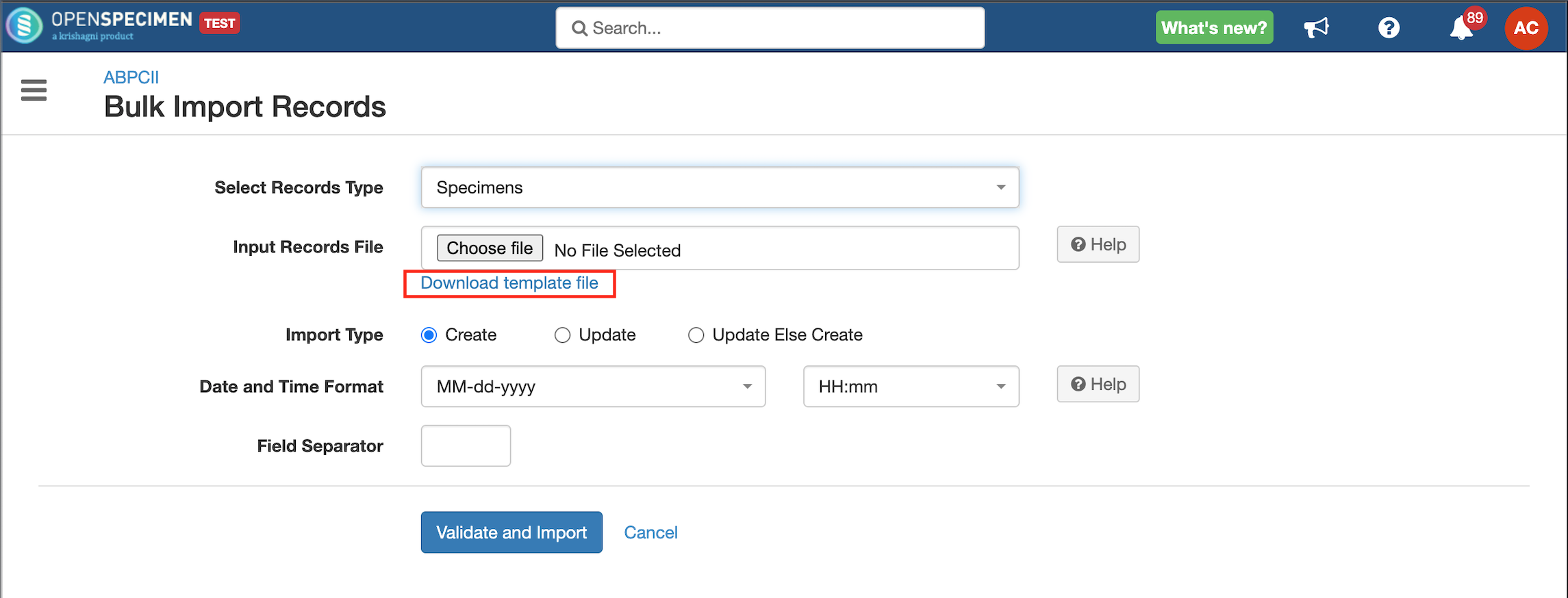
Steps to Import CSV
Sample CSV
Moving Form Data while Moving Specimens
Export the form data for the specimens being moved. Refer to the wiki page for more information on exporting form data.
Move the specimen using bulk import update.
Delete the older form records to avoid confusion. For this, you can run a bulk import update by using the older record ID and activity status as 'Disabled'.
You have to choose 'Create' as bulk import type to move the forms data since you are creating new form records under the second CP. Using the form data exported in the first step, remove the record ID column.
Note:
Ensure that the form is attached to the CP at the required level.
Data Dictionary
Column Name | Datatype | Mandatory? (Create) | Mandatory? (Update) | Description | Permissible Values | Validations |
|---|---|---|---|---|---|---|
| Identifier | Number | No | Depends | System auto-generated unique number for every specimen. | Mandatory while updating. | |
| CP Short Title | String | Yes | No | The collection protocol short title under which specimen is collected | ||
| Visit Name | String | Yes | No | The name of the visit under which the specimen has to be created. | Should be an existing visit name | |
| Specimen Requirement Code | String | No | No | Code defined in collection protocol for the corresponding specimen requirement. If not specified, specimens are created as unplanned specimens. For enabling CP encoding, please refer to the wiki page. | Should be a valid code defined in the CP. If blank, the specimen will be collected as unplanned. | |
| Specimen Label | String | Depends | Yes | Depending on the system setting, it should be unique within the system or CP. Mandatory when the auto label generation is OFF OR 'Manual Input' is set to 'Yes.' | ||
| Barcode | String | No | No | It should be unique within the system. | ||
| Class | String | No | No |
| ||
| Type | String | Yes | No | Permissible values for specimen class-type | ||
| Lineage | String | No | No |
|
| |
| Parent Specimen Label | String | No | No | Should exist within the same CP → Participant→Visit. Mandatory in the case of derivatives and aliquots. | ||
| Anatomic Site | String | No | No | Permissible values for anatomic site | Default value: 'Not Specified' | |
| Laterality | String | No | No |
| Default value: 'Not Specified' | |
| Collection Status | String | No | No |
| Default value: 'Collected' | |
| Pathological Status | String | No | No |
| Default value: 'Not Specified' | |
| Initial Quantity | Double | No | No | |||
| Available Quantity | Double | No | No | If not specified, it takes the same value as 'Initial Quantity.' | ||
| Concentration | Double | No | No | |||
| Biohazard | String | No | No | Multiple values can be specified as: Biohazard#1, Biohazard#2, Biohazard#3 and so on |
| |
| Freeze/Thaw Cycles | Integer | No | No | Number of freeze-thaw cycles | ||
| Created On | Date & Time | No | No | Needs to be entered for child specimens. | It takes the current date if left blank. Note: Created On date for child specimens is the processing date for parent specimens. | |
| Comments | String | No | No | |||
| Location#Container | String | No | No | Name of the container. It needs to be specified if the specimen is not virtually located. |
| |
| Location#Row | String | No | No | Position within the container at which the specimen is to be stored. If left blank, the system will allocate the next available position. | ||
| Location#Column | String | No | No | |||
| Location#Position | Integer | No | No | Linear position in its parent container | ||
| Collection Event#Date and Time | Date & Time | No | No | If left blank, it will take the current date and time. | ||
| Collection Event#Comments | String | No | No | |||
| Collection Event#Procedure | String | No | No | The procedure used during specimen collection |
| |
| Collection Event#Container | String | No | No | Type of container used for specimen collection |
| |
| Collection Event#User#Email Address | Email Address | No | No | Should be an existing user | ||
| Received Event#Date and Time | Date & Time | No | No | Select the correct date and time format from UI based on the data in the file. If left blank, it will take the current date and time. | ||
| Received Event#Comments | String | No | No | |||
| Received Event#Quality | String | No | No |
| ||
| Received Event#User#Email Address | Email Address | No | No | Should be an existing user | ||
| Activity status | String | No | No | Used to close or reopen the specimens in bulk |
|
|
Reason | String | No | No | Reason for closing the specimen | ||
Created By#Email Address | String | No | No | Email address of the user who processed the sample | Should be an existing user | |
External IDs#1#Name | String | No | No | Additional Identifiers source | ||
External IDs#1#Value | String | No | No | Additional IDs for specimen |
Use '##set_to_blank##' for the fields which need to be updated to blank.
Collection event and received event date/time fields of a collected specimen cannot be blanked out or set to empty. If you attempt to do that, no change is made. You can blank out dates for any custom field. (Make sure the form is attached to ALL CPs. Otherwise, it won't appear under the csv file.)