Table of Contents
In participant-centric studies, participant data like name, age, sex, etc., is collected along with the specimens. The study can be -
...
| Field Name | Mandatory | Description | Example |
|---|---|---|---|
Sites | Yes | You can select multiple sites from the dropdown where the study is being carried out. | Arkansas Hospital, HIGH Lab |
| Title | Yes | The long title of the research study | Cardiovascular diseases and risk factors knowledge and awareness in people with type 2 diabetes mellitus: a global evaluation |
Short Title | Yes | Short title to identify the study from the CP list page | Type 2 Diabetes Mellitus Study |
Code | No | 'CP code' that can be used to generate the participant IDs | DM |
Principal Investigator | Yes | The user who leads the study | Select value from the dropdown - Dr. Dan |
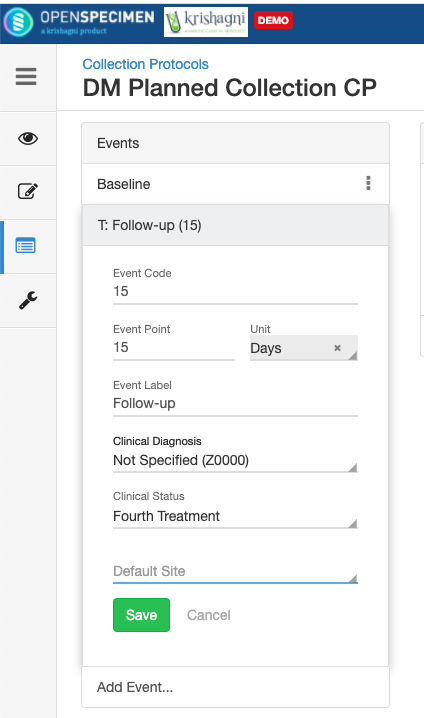
| Protocol Coordinator | No | A specialized a researcher who supports the management and coordination of clinical research studies. There can be multiple coordinators selected. | |
| Date | No | Start and End date of the study. Some clinical trials are for a specific duration. | |
| Ethics Approval ID/IRB ID | No | The 'approval ID' given by the Ethics committee to conduct the study | A656678 |
| Type | No | If the study collects participant's information, then select 'Participant centric' else 'Specimen centric' for specimen biobanks. | Participant/Specimen centric |
| Anticipated Participants Count | No | The number of participants that will be registered to the protocol. (The number of actual registrations can exceed) | 10000 |
| SOP Document URL | No | Link to the SOP document uploaded on the university web portal | https://university.edu/sop |
| SOP Document File | No | During CP creation, the user can also upload the study-related standard operating procedure document files. The users can access the SOPs that have been set at the CP level during data entry. This helps them perform any procedure consistently. | |
| Store SPRs? | No | The visit page will show the SPR tab if enabled. | Refer to Wiki page https://openspecimen.atlassian.net/wiki/x/bgB9 for more details |
| Extract Text from SPR PDF Docs? | No | The text from the uploaded SPR file is shown in the 'Surgical Pathology Reports' section when enabled. (If the SPR contains scanned images, then there is no text extracted) |
...
| Expand | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
|
...