| Table of Contents | ||
|---|---|---|
|
...
We are happy to announce that v7.0 is ready for download! As always, it has new features, improvements and bug fixes selected based on feedback from many of our current adopters.
New features highlights:
- eConsent module
- Mobile app
- Email survey forms to participants
- Bulk operations on participants & specimens via UI
- Display custom forms based on rules
- Email survey forms to patients
- Query Enhancements: Hide unwanted fieldsCreate derivatives & aliquots in a single workflow
- Separate access control to visits and specimens
- REDCap plugin improvements
- eConsent plugin
- Mobile App
- Many bug fixes and usability enhancements
Download
Version | Link | Questions |
|---|---|---|
| Enterprise | Contact support team | support@krishagni.com |
| Community | Github repository for v7.0(TBD) | forums.openspecimen.org |
...
Administrative Enhancements
...
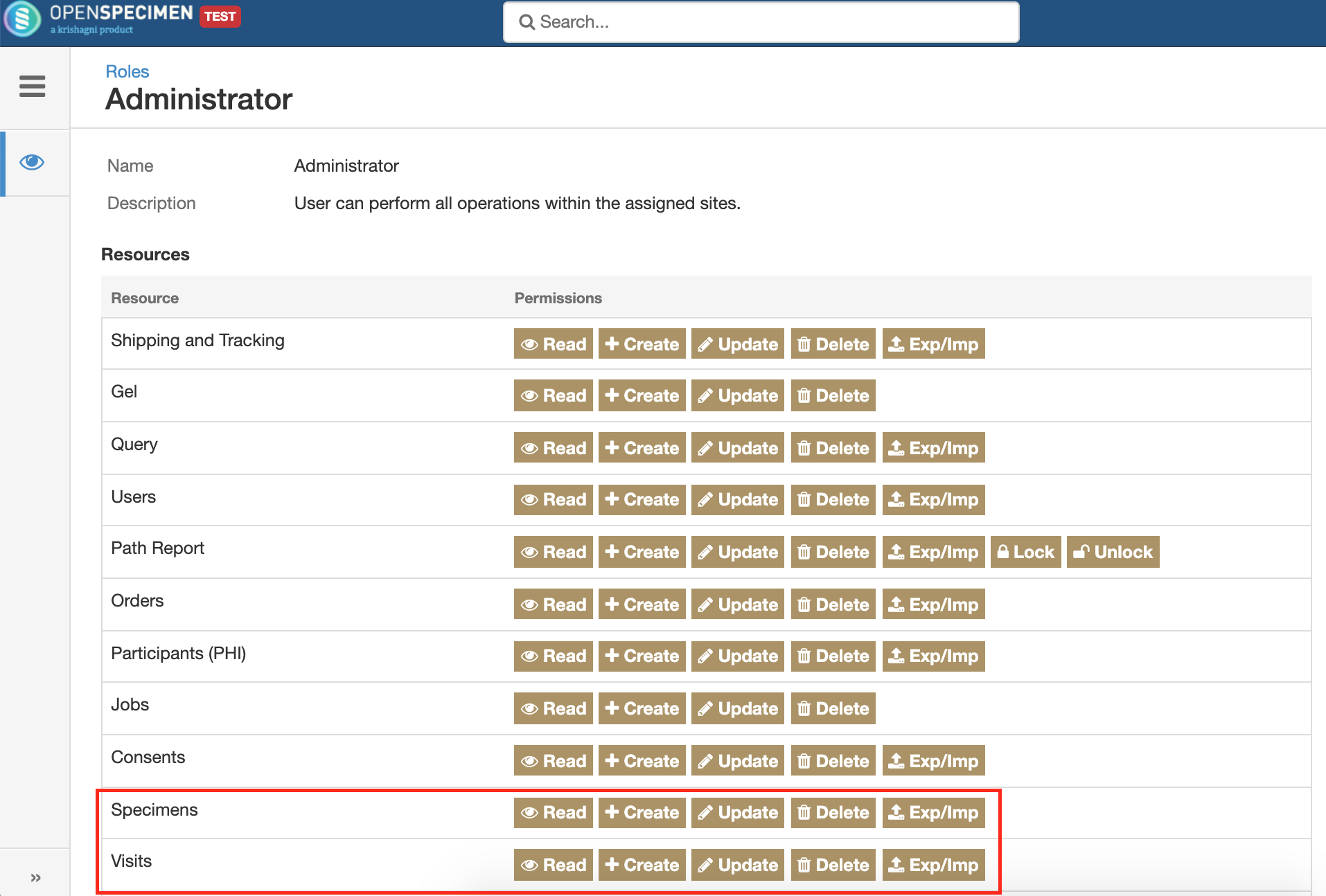
Separate roles for Visits and Specimens
The visits and specimens resources under roles have been separated out. This will allow users to be given access to visits but not any specimens. This is useful if clinicians are only handling participant and visits.
...
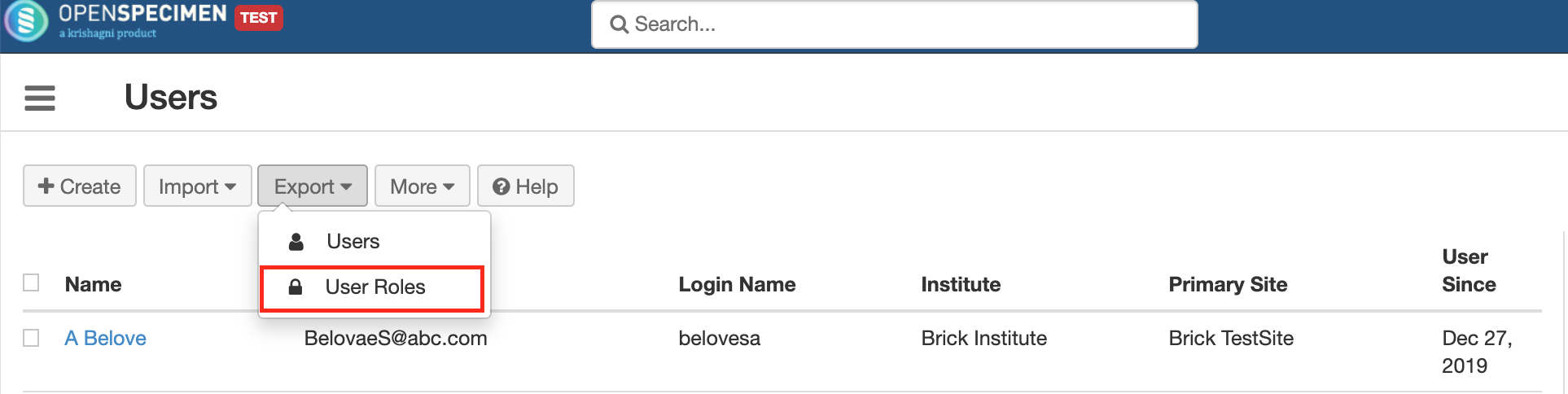
Export user roles
This helps in exporting user accounts and their roles to be imported to another server. For e.g. for moving user accounts from test to production server.
Bulk delete admin data
Using bulk operations, admins can now delete users, sites, institute and distribution protocols by setting activity status as 'Disabled'. Previously in this bulk templates, activity status column was missing. In v7.0, this column is included.
Collection protocol enhancements
- Administrators can now reopen closed events and requirements within a collection protocol
- Ability to delete collection protocol group
- Ability to close collection protocol so that no new registrations are not allowed
- JSON configuration to overwrite ordering of visits in the visits : table. By default visits are ordered by event timepoints but this allows to order by visit date.
Data entry enhancements
...
Bulk update specimens via UI
Multiple specimens can now be edited at once via user interface. This is now possible for any field of specimen including custom fields using the 'Actions' menu present in multiple places like cart, container specimens view, specimens list within collection protocol, visit overview page etc.
...
For more details, refer to 'Bulk update specimens via UI'.
...
Bulk update and delete participants
...
via UI
Multiple participants can now be updated or deleted via user interface from participants list within a collection protocol.
...
- Edit available quantity and freeze-thaw cycles while transferring specimens from one location to another.
- New specimen label token to include current date and time as part of the label
- Set system level visit name format via settings
...
New Modules
eConsents
OpenSpecimen's eConsents module helps clinical research centers to collect both types of consents - study-specific and broad-based consents. Below are the features of this module:
...
For more details, refer to 'eConsents'.
Email consent & survey forms to
...
participants
Forms (like consent forms, surveys, questionnaires, medical history, clinical history, etc.) can be collected directly from participants via email. Data gets recorded in OpenSpecimen once the patient clicks on the link and enters the data. This allows the patients to enter data at their convenience from anywhere.
...
All Improvements and bugs
Needs Jira login to view table.
| Expand | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
...